Initial comments on BfR recommendations for maximum levels for vitamins and minerals in food supplements by Food Supplements Working Group at the BLL (AK NEM)
- The Food Supplements Working Group at the BLL (AK NEM) comments on BfR's current proposals and recommendations for maximum levels for vitamins and minerals in food supplements in the following inital statement.
The German Federal Institute for Risk Assessment (BfR) has revised its 2004 recommendations on maximum levels for vitamins and minerals in food supplements (1, 2) on the basis of new scientific findings. The updated recommendations on maximum levels have been published in January 2018 (3). This was done, as BfR points out, in the light of the fact that no binding maximum levels have been set at European level to date. The proposed maximum levels are to serve as a decision-making aid for the risk management policy of the Federal Ministry of Food and Agriculture (BMEL) and to form the basis of legislation stipulating national maximum levels for vitamins and minerals in food supplements.
For years now, manufacturers of food supplements have been asking for statutory maximum levels for vitamins and minerals to be established to ensure that consumers and manufacturers throughout the European Union (EU) can benefit from (legal) certainty. Therefore, the resumption of the discussion on maximum levels as a result of the overdue update of the BfR recommendations on maximum levels is generally endorsed. At the same time, it should be noted that an open Europe-wide discourse is needed regarding the sometimes widely diverging interpretations of the scientific data and the different methodological approaches employed in the derivation of maximum levels, since only a uniform, Europe-wide determination of quantitative limits will lead to the desired outcome. Variations in maximum levels amongst EU member states, are not appropriate in terms of modern consumer protection legislation, given that consumers are able to shop across borders, and contravene the right to the free movement of goods in the EU. Against this backdrop, the Food Supplements Working Group at the BLL (AK NEM) issues the following initial comments on the BfR recommendations:
1. Statutory provisions and European context
The European legislator had almost reached this same point some ten years ago: following extensive discussions covering a diverse range of approaches, they were close to reaching agreement on concrete maximum limits of added vitamins and minerals in food supplements. Unfortunately, they failed to reach an agreement – not least because of the member states’ unwillingness to settle for a compromise.
Analysis of and comparison with other safety assessments needed
At the time, a model developed by scientific experts in cooperation with industry had been widely accepted by the European Commission and by the majority of member states (4). This model by Richardson (5) also incorporates the factors for upper safe levels of vitamins and minerals, intake quantities via normal nutrition and reference values for nutrient intake, in accordance with the parameters of European legislation (Article 5 (2) of the Directive 2002/46/EC (Food Supplements Directive)). Unlike the BfR model, however, it works consistently with real intake data and fact-based safety margins, which means that theoretical assumptions and conjecture can be dispensed with.
Using this model, and taking account of the latest current scientific findings, the proposals for maximum levels for vitamins and minerals in dietary supplements were revised by Professor Richardson as far back as 2014 (6). The results have been published and can contribute to a scientifically based discussion (7). Regrettably, an analysis of the content of this scientific study on the derivation of maximum levels cannot be found in the publication setting out the BfR’s latest recommendations on maximum levels, nor is there a substantive discussion of current safety assessments and recommendations by other member states.
The parameters handed down by the ECJ must be observed
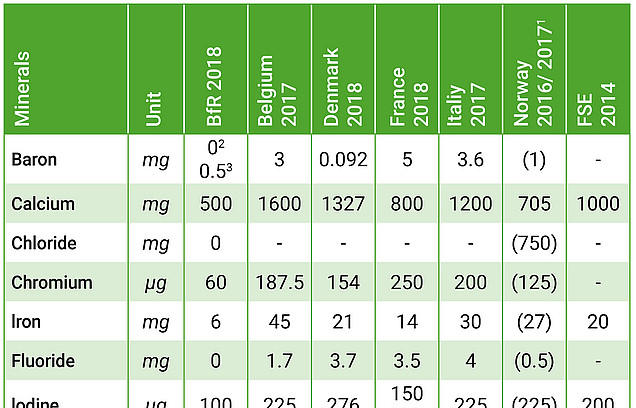
The absence of any discussion of current safety assessments by other European or global institutions and authorities in particular is surprising in the light of recent European Court of Justice (ECJ) rulings. In the reasons for its ruling on the French regulations for maximum levels of vitamins and minerals in food supplements (“Noria”, Case C-672/15), the ECJ argues that the criteria of Article 5 (1) and (2) of the Food Supplement Directive must be taken into account when setting maximum levels, with all reliable data, including international data, having to be included in the risk assessment. As the BfR states in its publication, a number of member states have recently introduced or updated maximum levels for vitamins and minerals. The overview illustrates the enormous differences in the maximum levels considered safe at the various national levels, based on current risk assessments. Particularly in terms of a Europe-wide comparison, it is apparent that the BfR proposals are extremely restrictive. The question arises as to how – in the absence of plausible scientific reasons regarding, for example, widely differing dietary habits – the interpretation of the same scientific data can lead to such divergent outcomes. This underscores the need for scientific and political discourse to take place at the European level.
Hypothetical risk no justification for prohibitions
As a rule, national provisions for maximum levels cannot prevent products containing higher doses, which are legally marketed in other member states of the European Union, to continue being lawfully sold in Germany in the same form. This would be contrary to the principle of mutual recognition. As the ECJ states in both the Noria and the Queisser rulings (Case C-282/15), a (higher dosage) product may only be rejected if there is a real, actual risk. Purely hypothetical assumptions regarding risks are not a sufficient justification for a ban. The fact that products conforming to the quantitative recommendations are “safe for people aged 15 years and over” on the basis of the present risk assessment according to the BfR does not therefore imply that higher-dosage products are to be regarded as unsafe.
2. Safety margins and multiple-exposure factors
The reason for the sometimes considerable discrepancies between the BfR recommendations on maximum levels and the maximum levels in other member states lies in the very conservative approach on which the BfR model is based, in that it incorporates several safety levels simultaneously.
For example, BfR has revised its recommendations on maximum levels not only on the basis of new scientific findings; it has also changed the target group without providing any additional justification for doing so. The derivation of maximum levels no longer refers to adults; it now refers to adolescents aged 15–19 years. As a result, the lower UL (upper safe levels) for adolescents (if available) are used for deriving recommendations on maximum levels.
Separation of derivation of maximum levels for adults and adolescents necessary
It is difficult to understand the reasoning behind this approach. Combining the derivation of maximum levels for healthy adults and for the more sensitive population group of adolescents is not sensible. Should there be a justified need to set separate maximum levels for this group of the population, then it ought to be done as an additional and separate step. Furthermore, consumer surveys have shown that the traditional consumer of food supplements is 50 years and older. According to a representative survey of German consumers, fewer than 2.5 per cent of users of food supplements were in the age group 18–24 (8).
Moreover, BfR is working with two additional tiers of general safety factors that are not based on scientific data or on real consumption or market data, but merely on theoretical assumptions. It should be noted in this context that the use of the two parameters “UL” and “intake of the German population group with the highest nutrient intake” already results in a double safety margin. By definition, the UL is a safe level for chronic (daily, lifelong) intake, and also already incorporates safety margins itself. Using the intake data of the German population group with the highest nutrient intake (95th percentile) through normal nutrition represents an additional safety factor, as most people have a lower intake in reality.
Using generalised multiple-exposure factors is scientifically questionable
BfR then goes on to incorporate two further safety levels for all nutrients in a generalised manner, by
- distributing the residual quantity that can be consumed in addition to the usual diet to food supplements and fortified foods, and
- including an uncertainty factor of 2 to account for possible multiple exposures to a nutrient through the intake of different food supplements.
BfR thus includes a factor of 4 for theoretical multiple exposure to many nutrients since the residual quantity as a difference between UL and the intake through traditional foodstuffs is ultimately divided by 4 in this model. In its derivation of safe maximum levels for the healthy German population, BfR therefore uses a hypothetical group of consumers which is supposedly consuming, in addition to their regular diet, 2 food supplements plus fortified foods on a daily basis and over a long period of time, i. e. chronically – all of which all contain the same added nutrient. There is no evidence that such an assumption corresponds to reality – quite the contrary. To declare multiple exposure as an independent risk factor, as the BfR does, is therefore difficult to reconcile with the requirements of the ECJ in the Queisser ruling (Case C-282/15). The existence of an actual (rather than merely hypothetical) risk cannot be argued on the basis of an assumption that is not borne out by reality. Furthermore, in 2004 the ECJ already rejected such a sweeping approach in its decisions Commission/Germany (Rs. C-387/99) and Commission/Austria (Rs. C- 150/00).
Generalised approach not backed by real consumption and market data
There is for instance no survey supporting the assumption that consumers use food supplements and fortified foods simultaneously and in large quantities and, above all, on a regular basis. Data on the nutritional intake of vitamins and minerals through the consumption of fortified foods is not available for Germany. What market analyses do show however, is that fortified foods make up only a small proportion of pre-packed foods in Germany (2016: 4.4 per cent) and, contrary to public perception, they do not represent a high-growth, dynamic market. On the contrary: the market for fortified foodstuffs as a whole has not recorded any growth in volume over the period covered in the most recent market analysis (2011 to 2016), a slightly positive trend in terms of turnover notwithstanding. The market share of vitamin-fortified packaged foods was likewise at a constant low level of around 2.5 per cent in 2016, with a slight downward trend. The fact that the share of the overall vitamin and mineral intake accounted for by fortified foods is regularly overestimated is not new (9), especially since a large part of the foodstuffs (unpackaged and unprocessed) is not accessible to enrichment in any way.
Neither does representative information on the use of food supplements in Germany justify a general multiple-exposure factor of 2 for the theoretical multiple intake of a nutrient through the consumption of different food supplements. Recent studies (10) confirm the results of previous studies (11) according to which only a small proportion of the population consumes several food supplements containing the same nutrients on a daily basis. Consumers prove to be quite responsible in their use of food supplements. Consumers do take heed of the information provided on the packaging and are aware of the potential risks associated with an excessive intake of nutrients (8). The fact that consumers act responsibly in their use of food supplements is underscored in the sense that if they do consume several products simultaneously at all, they make deliberate choices in combining them (10). “Multiple exposures”, i. e., the intake of a particular nutrient by simultaneously consuming several food supplements, was of little relevance. For 93.8 per cent of users of food supplements, the relevant nutrients are sourced from a single product. Thus, data about the multiple intake of food supplements containing the same nutrients is in fact available; the use of a general “uncertainty factor regarding an unknown but possible multiple intake of food supplements with the same nutrients” cannot therefore serve as justification. The publications cited above are mentioned elsewhere by the BfR, but an examination of these findings is missing in their paper.
Risk assessment must be based on real consumption patterns
Risk assessment must be based on real consumption patterns, rather than on assumptions or conjecture. The determination of the vitamin and nutrient intake in the risk assessment must therefore be based on representative consumption surveys. If a multiple exposure factor is to be applied at all, then this must be considered and justified separately for each nutrient.
3. Model calculation and individual case studies
In its latest publication, BfR has taken heed of some of the criticisms regarding the frequent rejection of its model, which had been expressed in relation to the derivation of the recommendations on maximum levels from 2004. In particular, their decision to dispense with a maximum limit for nutrients (vitamin B1, vitamin B2, biotin and pantothenic acid), for which no UL has been derived due to the absence of indications of adverse effects, is to be welcomed.
Data from other recognised institutions to be taken into account
However, it is not clear why, in the absence of UL derivations by SCF/EFSA, the upper intake levels published by other recognised institutions (IOM, EVM) were not used consistently in instances where the relevant data exist. This was only done in the case of iron. In particular, no justification is given for the non-use of these ULs in the case of nutrients to which the derivation procedure was otherwise applied (vitamin C, potassium, chromium). The lack of explanation of the reasons for not taking these values into account in the publication is regrettable, especially against the backdrop of the repeatedly formulated requirement (Food Supplement Directive, guidance paper of the European Commission 2007, ECJ ruling Noria) that risk assessments should be based on generally accepted data, and hence that the findings of other scientific bodies must also be taken into account.
It should be noted as a matter of principle that scientific discourse always becomes necessary when different recognised bodies produce strongly divergent UL derivations, as in the case of vitamin B6, vitamin E, potassium or manganese. After all, the UL serves as the starting point for any risk assessment. In Europe, the EFSA is the institution that would be asked or mandated to carry out a scientific (re-)evaluation of ULs.
4. Benefits and risk assessment
BfR states that the risk assessment must take into account not only the risks arising from an excessive intake but also those arising from an inadequate intake. In principle, the weighing of the risks against the benefits in the regulatory effort to set maximum limits is to be welcomed, particularly in view of the fact that, despite adequate provision at population level, the nutrient intake for individual population groups or individuals may be inadequate. For example, NVSII data shows that for almost all nutrients evaluated, a section of the population would benefit from an additional intake through food supplements. Among users of food supplements, the percentage of people who do not meet the reference values for vitamin D, E, C, folic acid, calcium and magnesium decreases by 6 to 25 per cent.
Separate benefit and risk assessment required
Combining the consideration of benefits and risks as part of a safety assessment is questionable on both scientific and legal grounds and does not lead to the desired result either. The assessment of the health risk associated with an additional intake and the discussion about the benefits of such an additional intake should be conducted separately. Neither perceived positive health effects nor an adequate nutrient supply at the level of the general population can serve as justification for restrictions. The establishment of maximum levels for food supplements is justified by the need to protect the consumer against adverse health effects (Recital 13 of Directive 2002/46/EC) – but not by nutritional needs. Furthermore, according to the case law of the European Court of Justice (ruling of 23/09/2001, C-192/01, Commission/Denmark), the absence of a nutritional need alone cannot justify a ban on the marketing of products lawfully manufactured and/or marketed in the other member states.
BfR nevertheless justifies some of its maximum level recommendations on the grounds that although the data regarding the safety assessment might not be conclusive, no positive health effects can be demonstrated for an intake above normal requirements (e. g. vitamin E), or that no reasons for an additional intake are discernible (e. g. phosphorus). Whether this approach meets the requirements of a science based risk assessment, especially in light of the fact that for some of these nutrients, EFSA and/or IOM have determined UL, and a derivation of maximum levels would have been possible on that basis, is highly questionable.
Risks for unhealthy population groups must be assessed separately
In this context, it should be emphasised once again that food supplements are intended for the general, healthy population. For the purpose of deriving maximum levels, it therefore does not make sense to take into account for instance people with chronic diseases or metabolic anomalies. It can generally be assumed that these people receive special instructions as part of their disease management and are therefore informed about possible interactions with foodstuffs and individual ingredients. If anything, consideration should be given to (warning) notices on products where there is a particular need to protect specific population groups as part of their risk management.
5. Additional aspect: the principle of proportionality
In its first discussion paper from 2006 (12), the European Commission already pointed out that, with regard to the manner in which safety is ensured, the primary-law obligations – including the principle of proportionality – must be complied with. This principle should be considered all the more at the national level, as an overly restrictive approach, ¬without adequate justification in the form of a safety risk for consumers, would lead to undue discrimination against domestic companies in the food supplements industry. If BfR's recommendations on maximum levels were to be implemented in the proposed form, German companies would have to expect a sharp decline in domestic and export sales.
No undue discrimination against domestic companies
Firstly, there would be the threat of a shift in demand towards higher-dose products which foreign suppliers would still be permitted to sell in Germany on the basis of the principle of mutual recognition. Furthermore, it is to be feared that consumers will increasingly purchase products over the Internet if they find that “their” products are no longer available on the market. This would clearly not be a desirable outcome from the point of view of consumer protection. While there are respectable online offers, it is well known that products are sold which are questionable with regard to their advertising, composition and dosage already today.
Secondly, German companies would be at risk of sustaining major losses in terms of exports, because exporting goods to some non-member countries requires special certificates documenting the marketability of the product in Germany, the so-called marketability or export certificates (free-sale certificates). Many non-member countries permit the marketing of products that are already approved for the European market. To this end, manufacturers must be able to prove that their products can actually be lawfully sold there. In Germany, for example, they can obtain such proof in the form of a certificate issued by the competent public authorities. It is to be feared that the authorities will in future only issue free-sale certificates for products containing the prescribed low levels of vitamins and minerals; as a result, German companies with low-dose products would no longer be competitive in many export markets.
6. Conclusion
The setting of maximum levels for vitamins and minerals in food supplements, as provided for by the European legislator, is to be welcomed not only from the point of view of consumer protection, but it also serves to ensure (legal) certainty for companies, and guarantees the free movement of goods in Europe. However, these objectives can only be achieved if the maximum levels: (i) are derived on a scientifically sound basis, (ii) implemented throughout Europe, and (iii) reflect the necessary proportionality.
For the reasons outlined above, the new BfR recommendations do not fully meet these criteria. In summary, the criticism is based on the following main points:
- Risk assessments must be based solely on scientifically justified grounds and must be fact-based. This means the assessments must be conducted using real consumption data and fact-based safety margins. Conducting risk assessments using generalized, scientifically unfounded safety factors is inconsistent with this approach.
- The derivation of maximum levels for vitamins and minerals for adults on the one hand and adolescents as well as other at-risk groups, if any, on the other, should be carried out separately.
- In accordance with current case law, all relevant scientific data must be included in the risk assessment. This also includes the scientific examination of existing recommendations, in particular those issued by other European or worldwide institutions and public authorities. The comparison with other European member states shows that BfR’s proposals are disproportionately restrictive.
- Safety is the primary consideration in the derivation of maximum levels. An overly restrictive approach that cannot be justified in terms of protecting the healthy adult population is disproportionate. It unnecessarily restricts the choice of products for consumers and puts the domestic food industry at disadvantage, both in the German market and in export markets.
- The scientific and political discourse must be conducted at the European level because it is indispensable that uniform maximum levels for vitamins and minerals are applicable throughout Europe. Differences in the maximum levels in different EU member states make no sense when consumers are free to shop across borders; they are neither appropriate for the times in which we live, nor are they useful in terms of consumer protection.
Sources
- BfR 2004a. Verwendung von Vitaminen in Lebensmitteln. (Use of vitamins in food-stuffs.) Published by Domke A, Großklaus R, Niemann B, Przyrembel H, Richter K, Schmidt E, Weißenborn A, Wörner B, Ziegenhagen R. BfR Wissenschaft 03/2004
- BfR 2004b. Verwendung von Mineralstoffen in Lebensmitteln. (Use of minerals in foodstuffs.) Published by Domke A, Großklaus R, Niemann B, Przyrembel H, Richter K, Schmidt E, Weißenborn A, Wörner B, Ziegenhagen R. BfR Wissenschaft 04/2004
- Weißenborn et al. 2018. Höchstmengen für Vitamine und Mineralstoffe in Nahrungsergänzungsmitteln (Maximum levels for vitamins and minerals in food supplements). J Consum Prot Food Saf; doi.org/10.1007/s00003-017-1140-y
- European Commission 2007. Orientation paper on setting maximum and minimum amounts for vitamins and minerals in foodstuffs. SANCO/E4/ FDA/bs D/540510, Brussels, Belgium
- Richardson 2007. Risk management of vitamins and minerals: a risk categorisation model for the setting of maximum levels in food supplements and fortified foods. Food Science and Technology Bulletin: Functional Foods 4 (6): 51–66
- Richardson 2014. Risk management approaches to the setting of maximum levels of vitamins and minerals in food supplements for adults and for children aged 4–10 years. http://www.foodsupplementseurope.org/publications-guidelines/
- Richardson 2015. School of Chemistry, Food and Pharmacy, University of Reading, UK. Risk analysis approaches for establishing maximum levels of essential nutrients in fortified foods and food (dietary supplements). In Science and the Law: How the communication of science affects policy development in the environment, food, health and transport section. Chapter 9, pp 153–173. Chapter DOI 10.1021/bk-2015-1207.ch 009. ACS Symposium Series, Vol. 1207, ISBN 13: 97808411231085. Copyright @ 2015 American Chemical Society
- Heinemann et al. 2015. Verwendung von Nahrungsergänzungsmitteln mit Vitami-nen und Mineralstoffen – Ergebnisse einer deutschlandweiten Verbraucherbefragung (Use of dietary supplements containing vitamins and minerals - Results of a Germany-wide consumer survey). J Verbr Lebensm; 2: 131-142
- Godfrey et al. 2004: The impact of fortified foods on total dietary consumption in Europe. British Nutrition Foundation Nutrition Bulletin 29: 188-198
- Willers J et al. 2015. Welche Bedeutung besitzt die Mehrfachverwendung von Nah-rungsergänzungsmitteln? (What is the significance of the multiple use of dietary supplements?) Findings of a Germany-wide consumer survey. J Verbr Lebensm; 2: 1-9
- Beitz et al. 2004. Vitamin- und Mineralstoffsupplementierung in Deutschland (Vitamin and mineral supplementation in Germany). Bundesgesundheitsblatt 47: 1057-1065
- European Commission 2006. Discussion Paper on the setting of maximum and minimum amounts for vitamins and minerals in foodstuffs. Brussels, Belgium
A German version can be accessed here:
- Stellungnahme des AK NEM bezüglich BfR-Empfehlungen zu Höchstmengen für Vitamine und Mineralstoffe in Nahrungsergänzungsmitteln
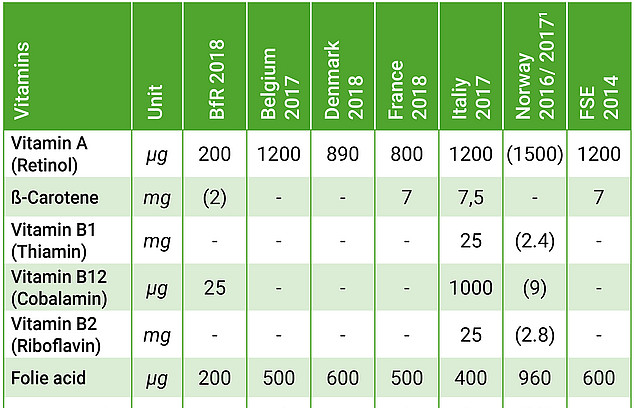
Overview of national maximum levels for vitamins.
Show Infographic